Wattage, Lumens, Measuring Irradiance, and Actinometry: All Critical
Light sources used in photochemical reactions are labeled and evaluated in several ways, but none of the corresponding numbers help to better understand how much light is absorbed by the reaction. This post explains the difference between wattage and lumens, the importance of measuring irradiance, and how actinometry is critical for evaluating light sources in photochemistry.
In photochemistry, light is a reagent. And no chemist performs a reaction without knowing the reagents’ stoichiometry. Accordingly, it is important for chemists to evaluate light sources in photochemistry the number of photons from the light source required for any given experiment. That one detail (the amount of light penetrating the sample) provides the requisite information to properly study, optimize, and scale any experiment. Unfortunately, too few publications document the photochemistry experiment’s light source. Even fewer publications specify the required number of photons for the reaction.1

Various Light Sources for Photochemistry
Continue reading to learn the most important considerations when evaluating light sources in photochemistry. We also discuss how to measure your light’s chemical energy or photon flux in your reactions.
Understanding electrical power vs light energy
Scientific publications typically describe photochemistry light sources in terms of color and power, usually wattage. But a light source’s electrical power rating (wattage) is only an indication of that light’s energy. However, LED and CFL light sources (as an example) do not have the same luminous efficacy. They don’t deliver the same amount of light to the reaction. Nearly all commercial light bulbs are rated in lumens. The lumen is “a measure of the total quantity ofvisible light emitted by a source per unit of time.” In other words, lumens represent visible light generated by the bulb. And while a luxmeter measures the light’s intensity (bright intensity) at a specific position (lux, measured in lumen/m2), monochromatic light sources (those used in photochemistry experiments) make these measurements irrelevant. The takeaway is clear: electrical wattage measure the light source’s energy, lumens measure the amount of visible light. However, neither are helpful in understanding how much light is absorbed by the reaction.
Light beam geometry
Another important consideration is the geometry of the light as it disperses around the sample. Regular bulbs (like those found in homes) diffuse light in every direction while focused light sources direct the light in one direction. As the chart below indicates, the light source’s beam angle directly impacts its intensity. A focused, or angled, light source with a much lower wattage is able to easily produce more intensity than a diffuse light source of much higher wattage.
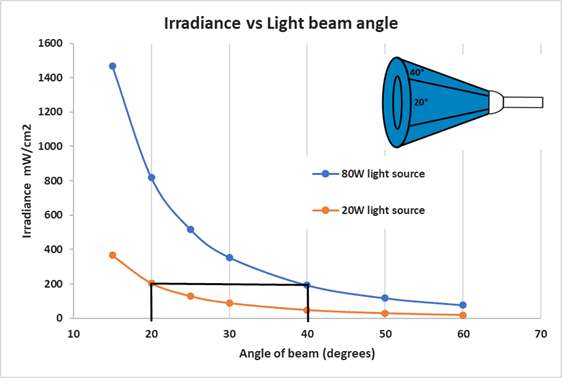
The y-axis represents the light intensity (irradiance) while the x-axis represents the beam’s angle. The chart above demonstrates that a 20 W LED light with 20 degrees of beam angle is as efficient as an 80W LED light with 40 degrees of angle
Therefore, there is a direct relationship between the beam’s angle and the wattage needed to produce the same intensity. It’s important to choose a light source that will focus the majority of its light directly on the sample. The wattage of the light source is less important. It should not be too small, so as it covers the entire sample. It should not be too large either, as light energy not focused on the sample is wasted.
Radiospectrometry
A radiospectrometer measures a light source’s power (radiant flux, in watt) and light intensity (irradiance in watt/cm2) as well as spectrum (nm). However, irradiance is different from lux and is not based on human eye sensitivity and visible light. Irradiance is measured at a specific position along a light’s source (coming from one direction). Additionally, it effectively compares different light sources in a standardized setup. Therefore, it is a better measurement for non-visible light sources (like near UV). But keep in mind that the light source’s sensor position effects the irradiance measurement. Comparisons are difficult if you don’t know the sensor’s exact location.
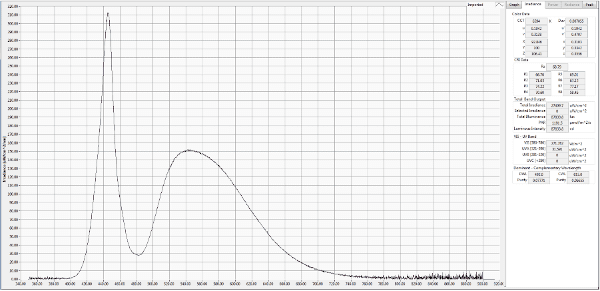
White LED Spectrum
If you place your sample in the same position you can estimate the amount of light (number of photons) that irradiates your sample using the exposed surface area of your sample. You need to take into consideration the light coming from other directions as well as the reflection of the light on the surface of the vial. Every HepatoChem light source is tested via radiospectrometry and the corresponding irradiance (listed as mW/cm2), as well as the specific light spectrum,is included with each light source.
Actinometry and the importance of calculating photon flux
Actinometry is a standard method to measure the actual amount light penetrating your sample or photon flux. “Actinometers” are the method’s reagents and Ferrioxalate is the most widely used.2 This iron (III) complex produces iron (II) in known photochemical yields. Thus, the specific number of photons penetrating the sample can be determined based on how much iron (II) is produced from the ferrioxalate complex. 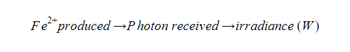 The photon flux (or irradiance) is specific to the vial, volume of the reaction and the light source. Using this method, you can calibrate your setup and know how much light penetrates your sample.
The photon flux (or irradiance) is specific to the vial, volume of the reaction and the light source. Using this method, you can calibrate your setup and know how much light penetrates your sample.
Click here to learn more about determining photon flux in your photochemistry experiments.
1. Bonfield, H.E., Knauber, T., Lévesque, F. et al. Photons as a 21st century reagent. Nat Commun 11, 804 (2020) https://doi.org/10.1038/s41467-019-13988-4
2. Hatchard C.G.; Parker C.A. A new sensitive chemical actinometer. 2. Potassium ferrioxalate as a standard chemical actinometer. Proc. R. Soc. London, Ser. A. 1956, 235, 518-536.

